Lewis Structures
530 likes | 1.57k Vues
Lewis Structures. Lewis Structures At the conclusion of our time together, you should be able to:. List the basic rules for drawing a Lewis Dot structure for a compound Use these rules to draw a Lewis Dot structure for a compound. Another New Element On The Periodic Table

Lewis Structures
E N D
Presentation Transcript
Lewis StructuresAt the conclusion of our time together, you should be able to: List the basic rules for drawing a Lewis Dot structure for a compound Use these rules to draw a Lewis Dot structure for a compound
Another New Element On The Periodic Table Element: Man Symbol: Xy AtomicMass: 180 +/-100 CommonName(s): Varies anywhere from John to !@#$&*!
In 1916 G. N. Lewis proposed that atomscombine in order to achieve a more stableelectron configuration. Maximum stability results when an atomis isoelectronic with a noble gas. An electron pair that is shared between two atoms constitutes a covalent bond. The Lewis Model of Chemical Bonding
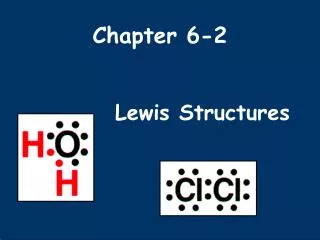
Covalent Bonding in H2 Sharing the electron pair gives each hydrogen an electron configuration analogous to helium. . . H H : H H Two hydrogen atoms, each with 1 electron, can share those electrons in a covalent bond.
Covalent Bonding in F2 Sharing the electron pair gives each fluorine an electron configuration analogous to neon. .. .. : : F F . . .. .. : : : F F .. .. Two fluorine atoms, each with 7 valence electrons, : : can share those electrons in a covalent bond.
The Octet Rule .. .. : : : F F .. .. In forming compounds, atoms gain, lose, or share electrons to give a stable electron configuration characterized by 8 valence electrons.
Xy Continued: Usage of Xy: None really, except methane production. Good samples are able to produce large quantities on command. PhysicalProperties: Solid at room temperature, but easily gets bent out of shape. Fairly dense and sometimes flaky. Difficult to find a pure sample. Due to rust, aging samples are unable to conduct electricity as easily as young, fresh samples.
.. . . . : C . F . .. : : F .. .. .. : : : F F C : .. .. .. : : F .. Example Combine carbon (4 valence electrons) andfour fluorines (7 valence electrons each) : to write a Lewis structure for CF4. The octet rule is satisfied for carbon and each fluorine.
.. : : F .. .. .. : : : F C : F .. .. .. : : F .. : : F .. .. : : C F F .. .. : : F .. Example It is common practice to represent a covalentbond by a line. We can rewrite .. as
.. .. .. .. : : : : : : : : C C O O O O : : : : : : H H C N C N Inorganic examples Carbon dioxide Hydrogen cyanide
H H H H .. .. : : : : H H C C C C H H : : : : : H H H H C C C C Organic examples Ethylene Acetylene
Back to Xy ChemicalProperties of Xy: Attempts to bond with Xx (Woman) any chance it can get. Also, tends to form strong bonds with itself. Becomes explosive when mixed with Kd (Kid) for a prolonged period of time. Neutralized by saturating with alcohol. Caution: In the absence of Xx (Woman), this element rapidly decomposes and begins to smell.
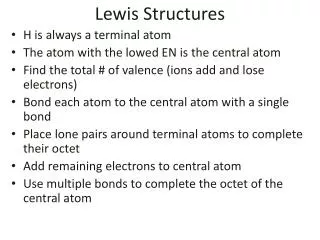
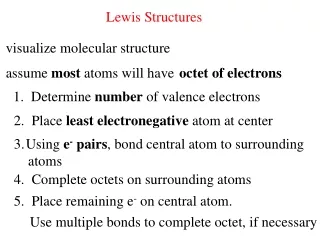
Rules for Lewis Structures 1. Make certain that the bond is a covalent bond then set up the skeleton structure as follows: The atom with the lowest electronegativity will tend to go in middle Place all the other atoms around this central atom Attach these atoms to the central atom in reasonable fashion with single bonds
Rules for Lewis Structures 2. Sum valence electrons 3. Complete octets of peripheral atoms 4. Place leftover e- on central atom 5. If necessary use multiple bonds to fill the center atom's octet.
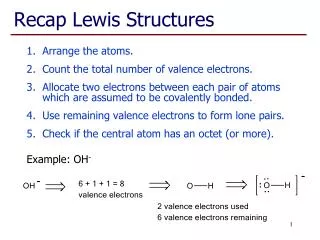
For NF3 Like NBr3 Molecular formula Atom placement Sum of valence e- : : N 5e- : F : : F : : F 7e- X 3 = 21e- N Remaining valence e- : F : : Total 26e- Lewis structure
PROBLEM: Write a Lewis structure for CCl2F2, one of the compounds responsible for the depletion of stratospheric ozone. PLAN: Follow the steps outlined previously SAMPLE PROBLEM: Writing Lewis Structures for Molecules with One Central Atom SOLUTION: Step 1: Carbon has the lowest EN and is the central atom. The other atoms are placed around it. Cl Cl C F F
: Cl : : : : Cl C F : : : : : : : F SOLUTION: Steps 2-4: C has 4 valence e-, Cl and F each have 7. The sum is 4 + 4(7) = 32 valence e-. Make bonds and fill in remaining valence electrons placing 8e- around each atom.
Writing Lewis Structures for Molecules with Multiple Bonds. SAMPLE PROBLEM: PROBLEM: Write Lewis structures for the following: Nitrogen (N2), the most abundant atmospheric gas PLAN: If an atom does not have an octet, Step 5which follows the other steps in Lewis structure construction must be done. If a central atom does not have 8e-, an octet, then an e- can be moved in to form a multiple bond.
: : N N . . : : N N N N : : . . . . SOLUTION: N2 has 2(5) = 10 valence e-.
7th Grade Science Answers "Blood flows down one leg and up the other."
Lewis StructuresLet’s see if you can: List the basic rules for drawing a Lewis Dot structure for a compound Use these rules to draw a Lewis Dot structure for a compound
Rules for Lewis Structures 1. Make certain that the bond is a covalent bond then set up the skeleton structure as follows: The atom with the lowest electronegativity will tend to go in middle Place all the other atoms around this central atom Attach these atoms to the central atom in reasonable fashion with single bonds
Rules for Lewis Structures 2. Sum valence electrons 3. Complete octets of peripheral atoms 4. Place leftover e- on central atom 5. If necessary use multiple bonds to fill the center atom's octet.
How many lone pairs of electrons surround the central atom in water H2O? • 1 • 2 • 3 • 4 • 0