Periodic Properties
140 likes | 283 Vues
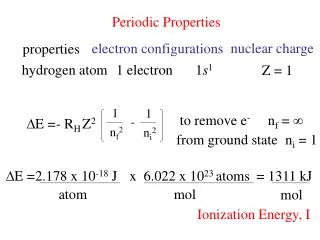
Periodic Properties. nuclear charge. electron configurations. properties. hydrogen atom. 1 electron. 1 s 1. Z = 1. 1. 1. to remove e -. n f = ∞. -. E =. - R H. Z 2. n f 2. n i 2. from ground state. n i = 1. E =. 2.178 x 10 -18 J. x 6.022 x 10 23 atoms. = 1311 kJ.

Periodic Properties
E N D
Presentation Transcript
Periodic Properties nuclear charge electron configurations properties hydrogen atom 1 electron 1s1 Z = 1 1 1 to remove e- nf = ∞ - E = - RH Z2 nf2 ni2 from ground state ni = 1 E = 2.178 x 10-18 J x 6.022 x 1023 atoms = 1311 kJ atom mol mol Ionization Energy, I
+ 2+ H 1s1 Z = +1 E = 1311 kJ/mol He+ 1s1 Z = +2 E = 5250 kJ/mol - - 1 1 = 5250 kJ/mol - E = - RH Z2 nf2 ni2 higher nuclear charge lowers orbital energy stabilizes system systems with more than 1 electron studied experimentally ionization reactions
- 2+ 2+ 1. Effect of 2 electrons in same orbital He+ 1s1 E = 5250 kJ/mol He 1s2 E = 2372 kJ/mol same nuclear charge Z = +2 orbital energy higher - e- e- repulsion - less stable easier to remove e-
2s 2s 1s 1s 3+ 3+ 2. Effect of electrons in different orbital Li ground state 1s2 2s1 E = 520 kJ/mol excited state 2s1 Li2+ E = 2954 kJ/mol same nuclear charge Z = +3 - - - - Zeff < Z inner electrons shielding charge
2s 2p 1s 1s 3+ 3+ 3. Effect of orbital shape Li ground state 1s2 2s1 E = 520 kJ/mol excited state 1s2 2p1 Li E = 341 kJ/mol same nuclear charge Z = +3 - - - - - - s orbitals penetrating lower energy
Electrostatic interactions determine orbital energies 1. Greater nuclear charge (Z) lowers energy electrons more difficult to remove 2. Electron-electron repulsion raise energy electrons easier to remove electrons shield Z inner electrons shield better 3. Orbitals with more penetration lower energy electrons more difficult to remove s < p < d < f
Ionization Energy energy required to remove an e- from gas phase atoms X (g) X+(g) + e- first ionization energy I1 X+ (g) X2+(g) + e- second ionization energy I2 lowest I1 Cs n = 6 He highest I1 n = 1
I1 increase Z increases shielding stays same adding valence e- core e- unchanged I1 decrease Zeff decreases more shielding e- core e-
2p 2p 2s 2s 1s 1s 5+ 10+ - - - - - - - - - - - - - - - B Ne
2p 2s 2s 1s 1s 4+ 5+ - - - - - - - - - B Be N O e- e- repulsion
Second Ionization Energy I2 I1 I7 I2 I3 I4 I5 I6 4560 Na 495 Mg 735 1445 7730 580 1815 11,600 Al Si 780 1575 16,100 P 1060 1890 21,200 S 2260 1005 27,000 very difficult to remove Cl 1255 2295 core electrons Ar 1527 2665
Atomic Radius 143 pm Al metallic radius Cl 100 pm covalent radius C-Cl 177 pm Cl 100 pm 77 pm C
Atomic Radius increase in size n dominates decrease in size Zeff dominates
- + Ionic sizes e- isoelectronic series same # electrons 46 e- ions get smaller +49 +50 +51