Section 2.3 Carbon Compounds
230 likes | 643 Vues
Section 2.3 Carbon Compounds. Organic Chemistry. Organic Chemistry- Study of all compounds that contain bonds between carbon atoms. I. Chemistry of Carbon. Characteristics of Carbon: 1. Carbon atoms have 4 valence electrons

Section 2.3 Carbon Compounds
E N D
Presentation Transcript
Organic Chemistry • Organic Chemistry- Study of all compounds that contain bonds between carbon atoms
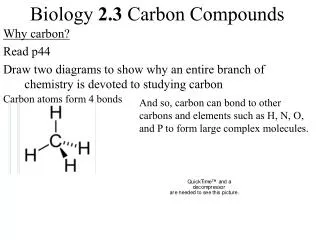
I. Chemistry of Carbon • Characteristics of Carbon: 1. Carbon atoms have 4 valence electrons 2. Each electron can join with an electron from another atom to form a strong covalent bond 3. Carbon can bond with many elements such as • Hydrogen • Oxygen • Phosphorus • Sulfur • Nitrogen SPONCH
Characteristics of Carbon 4. Carbon atoms can bond to other carbon atoms forming chains that are almost unlimited in length (macromolecules) 5. Carbon-carbon bonds can be • Single (C-C) • Double (C=C) • Triple (C C)
Characteristics of Carbon • Chains of carbon can even form rings • No other element has the versatility as carbon!
Macromolecules Macromolecules- large molecules made from 1000s or 100s of 1000s smaller molecules • Made by a process of polymerization • Made of smaller units called monomers joined together to form polymers (the monomers can be identical or different!)
Organic compounds or biomolecules are classified into 4 groups: • Carbohydrates • Lipids • Nucleic Acids • Proteins
1. Carbohydrates • Living things use carbohydrates as their main source of energy and structural purposes • The breakdown of sugars, such as glucose (C6H12O6) supplies immediate energy for all cell activity
1. Carbohydrates • Extra sugar is stored as complex carbohydrates known as starches • Monosaccharide- single sugar molecules such as glucose, galactose (milk), and fructose (fruits) • Disaccharide-two monosaccharides linked together
Polysaccharide-many monosaccharides joined together Ex. Glycogen- animal starch released from your liver when glucose in blood runs low Cellulose- plant starch which is tough and flexible. Major component of wood and paper
2. Lipids Lipids- fats, oils, and waxes • Compounds made mostly from carbon and hydrogen • NOT soluble in water! • Used to store energy
2. Lipids • Serves an important role in biological membranes and waterproof coverings • Many lipids are formed when a glycerol molecule combines with compounds called fatty acids
2. Lipids • Saturated-carbon atom in a lipids fatty acid chain is joined to another carbon atom by a single bond (maximum number of hydrogens!) • Unsaturated- at least one carbon-carbon double bond in a fatty acid (ex. Olive oil) • Polyunsaturated- fatty acids contain more than one double bond (ex. Cooking oils such as corn, sesame, canola and peanut oils)
3. Nucleic Acids • Nucleic Acids- Macromolecules that contain hydrogen, oxygen, nitrogen, carbon and phosphorus • Nucleotide- individual monomer consisting of a 5-carbon sugar, a phosphate group, and a nitrogenous base (A, T, C, or G)
3. Nucleic Acids • Individual nucleotides can be joined by covalent bonds to form a nucleic acid • Nucleic acids store and transmit heredity or genetic information • DNA- deoxyribonucleic acid (sugar=deoxyribose) • RNA-ribonucleic acid (sugar = ribose)
4. Proteins • Macromolecules that contain nitrogen, carbon, hydrogen, and oxygen • Monomers are called amino acids • Amino acids are compounds with an amino group (-NH2) on one end and a carboxyl group (-COOH) on the other end
4. Proteins • There are more than 20 different amino acids found in nature • Amino acids are joined by covalent bonds • The instructions for arranging amino acids into many different proteins are stored in DNA
4. Proteins 1. Proteins have a specific role • Control rate of reactions (enzymes) • Regulate cell processes (hormones) • Transport substances into or out of cells • Help fight disease (antibodies) • Form bones and muscles
4. Proteins 2. Proteins may have up to 4 levels of organization 1st- sequence of amino acids in a protein chain 2nd- amino acids can be twisted or folded 3rd- chain is folded * Van der Waal’s forces and hydrogen bonds help maintain the shape of the protein
III. Enzymes Enzymes- biological catalysts that cells use to speed up chemical reactions within a cell • Enzymes speed up reactions by lowering the activation energy • Activation Energy- the amount of energy needed to initiate a chemical reaction • Most enzymes work best at a certain pH and certain temperatures
Enzymes • Enzymes play essential roles in • Regulating chemical pathways • Making materials that cells need • Releasing energy • Transferring information
Red = without enzyme Green = with enzyme