Understanding the Periodic Table: History, Structure, and Element Properties
540 likes | 673 Vues
Explore the fundamental aspects of the periodic table, from its historical development to its modern interpretations. Learn how pioneering scientists like John Dalton, Dmitri Mendeleev, and Henry Moseley contributed to our understanding of atomic structure and periodicity. Delve into color coding, types of elements, and how to identify their properties. This educational packet aims to clarify elements' classifications, including metals, non-metals, and noble gases, while preparing you for assessments with helpful memory aids and structured activities.

Understanding the Periodic Table: History, Structure, and Element Properties
E N D
Presentation Transcript
Today: • Start of Unit 6: Periodic Table • 1) How the PT was put together • 2) Overview of the PT • 3) Color-coding the PT • 4) Work in packet - should be able to do through part C
Review of the Homework • John Dalton • Atom is the simplest form • Atoms of the same element have the same properties • Atoms combine or separate during chemical changes • Atoms cannot be destroyed
Prout • Suggested that all other elements develop from hydrogen • Why? Because all the atomic masses seem to be multiples of hydrogen • 1st hydrogen was the standard, then oxygen, and now it is carbon
Dobereiner’s Triads • Noticed that the certain groups of 3 elements have similar properties, and that the one in the middle seemed to be the average of the other 2
Nelands’ Law of Octaves • Suggested that when elements are listed in order of increasing atomic mass the first and 8th elements have related properties • Worked for what they knew then: • Undiscovered elements like noble gases
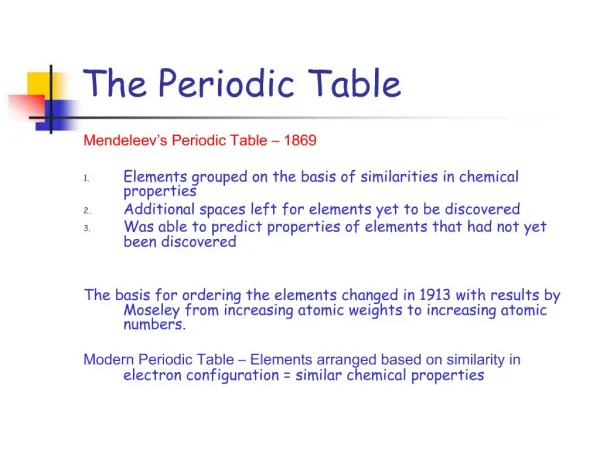
Mendeleev / Meyer • Mendeleev studied the chemical properties • Meyer studied physical properties • Both concluded that the properties of elements regularly repeated based on the atomic mass
Moseley • This guy used X-ray diffraction to measure the atomic numbers of elements • Revised Mendeleev’s table and fixed solved some of the mysteries that came with it • Groupings based on masses had errors • Groupings based on atomic number didn’t • Changed the law: Properties are periodic functions of ATOMIC NUMBERS
History of how it was put together • While watching the videos, fill in the first part of your packet • Video one • Video two
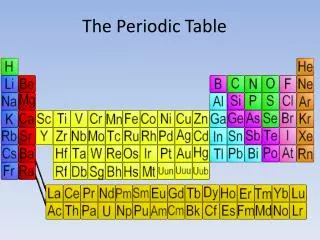
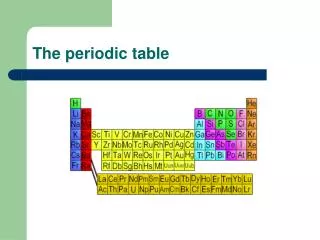
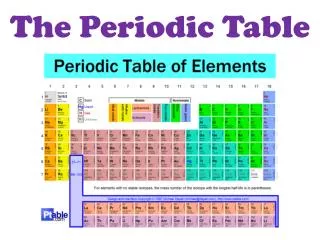
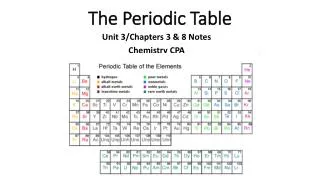
Overview of the Periodic Table • Developed by Russian scientist Dimitri Mendeleev (1869) • Revised based on the work of Henry Mosley (1913) and Glenn Seaborg (1940’s)
Revised or ‘Modern Periodic Law’ • The properties of the elements are periodic functions of their ATOMIC NUMBER. This means: • Elements are arranged by increasing atomic number • Predictable and repeating pattern to the elements properties
Modern Periodic Law • Elements in the same horizontal row (period) have the same number of energy levels. • The period number on the side of the PT is the same as the number of energy levels • Period 1: 1 energy level • Period 4: 4 energy levels
Modern Periodic Law • Elements in the same vertical column (group) have similar chemical properties due to the same number of electrons in the outermost energy level. In the electron configuration, the number of electrons in the outermost energy level is the number on the left RIGHT of the configuration for the tall groups
Modern Periodic Law • Group 1: 1 electron in the outermost energy level • Group 2: 2 electrons in the outermost energy level • Group 14: 4 electrons in the outermost energy level • Group 17: 7 electrons in the outermost energy level • *the ones place is the # of valence electrons
Types of Elements • Metals • Far left (except for H) • Non-metals • Right side • Metalloid • ‘The stairs’ between M and NM except “Al Po” the dog food • Noble Gases • Far right
Types of elements • Group 1: Alkali • Group 2: Alkaline Earth • Groups 1 and 2 are so REACTIVE (unstable) these elements are always found in compounds and never alone
Types of elements • Group 3-11: transition elements • Solutions of compounds containing these are usually colorful • CuSO4- Blue • CoCl2- pink
Types of elements • Group 17: Halogens • Has elements which are found in all three phases of matter (s,l,g)
Types of elements • Group 18: Noble Gases • Very stable due to having 8 electrons in the outermost energy level, except for He which has 2 electrons
Color Coding the PT • Other reminders: Gases are red on my PT • Liquids only: Br (NM) & Hg (M) • Memory Hook “Br I Cl N H O F” or end in GEN-INE • Elements that come in pairs • How do you remember the gases?? • This will be helpful come test time. Follow the steps as they are described in the packet. • Finish early? • Work on the work packet, should be able to get through part C
DO NOW • Take out your note packets • PT Riddle pHun • 8 minutes to finish and turn in
Types Of Elements Metals: • Found on the left side of the Periodic Table • About 2/3 of all the elements • Malleable: pressed thing • Ductile: able to be drawn into thin wires • Luster: Shiny!
Metals • Phase at 25oC: Solid, Except for Mercury • Silvery-white in color • Except for Cesium, gold & copper
Metals: Chemical Properties • Low Electronegativity • Lose electrons when bonding • Form + charge ions called cations • Low Ionization energy • Tend to tarnish in the presence of oxygen • Group 1 and 2 react violently with water
Nonmetal: Physical • Right Side of the PT • Brittle: can be crushed into a powder • Dull • Poor conductors of heat and electricity • Small atomic radius • Most are solids at room temperature • Only Liquid: Bromine;Gases: ‘Gen’s and ‘Ine’s: • Hydrogen, nitrogen, oxygen, fluorine, and chlorine • (excludes iodine- solid)
Nonmetal; Chemical • High Electronegativity • Gain electrons when bonding to form ionic bonds • From (-) charged ions called anions • Share electrons with other NM’s to form covalent bonds • Form molecules when bonded to other nonmetals • Group 17 NMs (halogens) are extremely corrosive • High Ionization energy • Ion name has ‘ide’ ending
Metalloid • Found along ‘the crack’ of the periodic table except for Al and Po (like the dog food) which are metals • A full side must touch the crack
Metalloid: Physical Properties • Sometimes conduct electricity (semiconductors) • Used to make computer microchips • Some have luster (like metals) • Brittle (like NM) • Photovoltaic (produce electricity from light) • Used to make solar energy panels • Solid at 25oC
Metalloid: Chemical Properties • Gain electrons from metals • Lose electrons to nonmetals
Noble Gas: physical properties • Low boiling points • All gases at room temperature • Poor conductors of heat and electricity
Noble Gas: Chemical Properties • Completely chemically nonreactive • No electronegativity (no bonds form) • Extremely high ionization energy
f) IMPORTANT DEFINITIONS • Electronegativity: the tendency of an atom to attract electrons in a bond • Found on ref. table s. • For metals it tends to be LOW • For NM it tends to be HIGH
Do now • Write down 2 characteristics of your sample
f) IMPORTANT DEFINITIONS • Ionization energy: energy required to remove the outermost electron • Found on RT. S • For metals it tends to be low • For nonmetals it tends to be high
f) IMPORTANT DEFINITIONS • Atomic Radii: The measure of the size of an atom • Found on RT. S • Radii decreases as you go across the PT left to right • Radii increases as you go down the periodic table
f) IMPORTANT DEFINITIONS • Reactivity: how easily they react • As a metal: how easily electrons are lost • Most reactive metal = Fr (and Cs) • As a nonmetal: how easily electrons are gained • Most reactive nonmetal: F • *Remember, the two F’s (F and Fr) are the most reactive!
g) Forming Ions: • An ionic bond forms when a metal loses valence electrons to a nonmetal forming a positive ion and the nonmetal, which gained the electrons forms a negative ion
g) Forming Ions: • These oppositely charged ions then attract to each other. By losing or gaining electrons each ion formed gest 8 valence electrons
Forming Ions • Metals lose valence electrons when forming positive ions called Cations • # electrons lost = positive charge of cation • This is known as oxidation Cute drawing to remember cation:
Bohr diagrams for Lithium • Neutral Ion
Forming Ions • What happens to the size of the metal atom when it forms a (+) ion? • Lets think about this… • Losing electrons… • Less than the neutral… • What would happen to the size of anything if part of it was lost… • DECREASES!!!!
Forming Ions • This is known as the ionic radius • The ionic radius for a metal ion is less than its neutral atom • (lose weight, get a smaller waist, lose electrons get smaller!)
Forming Ions • Nonmetals gain valence electrons when forming negative ions called anions • # electrons = negative charge • This is known as reduction • (The charge is reduced…) • Cute drawing to remember anion:
Fluorine Diagrams • Neutral Ion
Forming Ions • What happens to the size of the nonmetal atom when it forms a (-) ion? • Hmm… • Gains electrons… • Like gaining weight…. • What happens to the size…. • GETS BIGGER
Forming Ions • This is known as the ionic radius • Ionic radius of a nonmetal ion is greater than its neutral atom. • GAIN electron- GET BIGGER!
Complete the trends using your notes-we will then review the Reason Do whole chart