The Periodic Table
350 likes | 550 Vues
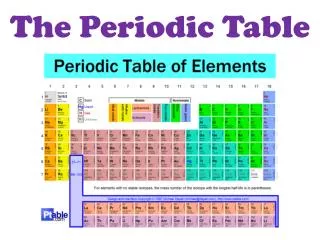
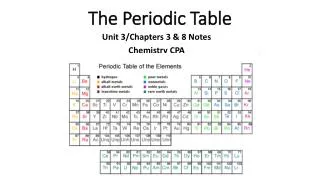
The Periodic Table. Unit 6. Why is the Periodic Table important to me?. The periodic table is the most useful tool to a chemist. You get to use it on every test. It organizes lots of information about all the known elements. Pre-Periodic Table Chemistry …. …was a mess!!!

The Periodic Table
E N D
Presentation Transcript
The Periodic Table Unit 6
Why is the Periodic Table important to me? The periodic table is the most useful tool to a chemist. You get to use it on every test. It organizes lots of information about all the known elements.
Pre-Periodic Table Chemistry … …was a mess!!! There were at least 55 elements discovered No organization of elements. Varying properties amongst the elements, but some similar trends Difficult to find information.
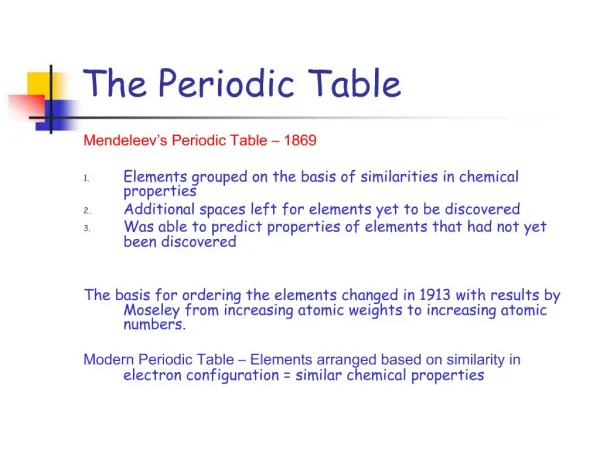
Dmitri Mendeleev In 1869 he published a table of the elements organized by increasing atomic mass. 1834 - 1907
Mendeleev’s Periodic Table • Mendeleev looked at all known elements and began to arrange them • Studied • Number of valence electrons • Similar chemical and physical properties • Atomic weight
Arranged elements in horizontal rows (periods) and columns (groups or families) Left holes where discovered elements did not fit
Dmitri Mendeleev: Father of the Table HOW HIS WORKED… Put elements in rows by increasing atomic weight. Put elements in columns by the way they reacted. stated that if the atomic weight of an element caused it to be placed in the wrong group, then the weight must be wrong. (He corrected the atomic masses of Be, In, and U) was so confident in his table that he used it to predict the physical properties of three elements that were yet unknown.
After the discovery of Sc, Ga, and Ge between 1874 and 1885, and the fact that Mendeleev’s predictions for their properties were amazingly close to the actual values, his table was generally accepted.
Henry Moseley In 1913, through his work with X-rays, he determined the actual atomic number of the elements. He rearranged the elements in order of increasing atomic number (number of protons). His research was halted when the British government sent him to serve as a foot soldier in WWI. He was killed in the fighting by a sniper’s bullet, at the age of 28. Memory trick: Mos Pros Modern Periodic Table = atomic number 1887 - 1915
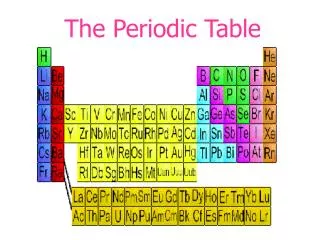
The horizontal rows of the periodic table are called PERIODS.
Atoms in the same group have similar chemical properties because they have the same number of VALENCE ELECTRONS! The vertical columns of the periodic table are called GROUPS, or FAMILIES.
Nonmetals to the right of the zig zag line Metals to the left of the zig zag line
Characteristics of Elements Metals Shiny when smooth and clean Solid at room temp. Good conductors of heat and electricity Malleable Ductile • Nonmetals • Generally gases or brittle, dull-looking solids • Poor conductors of heat and electricity • Metalloids • – Have physical and chemical properties of both metals and nonmetals!
Alkali Metals _s1
ALKALI METALS • Group1; 1 valence electron (one electron in outer orbital), very reactive metals • malleable, ductile, good conductors of heat and electricity. • softer than most other metals • can explode if they are exposed to water
ALKLINE EARTH METALS • Group 2; two valence electrons • metals • very reactive; not quie as reactive because has to lose two electorns in outer orbital to combine • not found free in nature
Transition Metals d - block
TRANSITION METALS • Can’t easily tell how many valence electrons; • ductile and malleable, and conduct electricity and heat • iron, cobalt, and nickel, are the only elements known to produce a magnetic field.
InnerTransition Metals These elements are also called the rare-earth elements. f block
RARE EARTH ELEMENTS • many areman-made; not necessarily rare • Used in metallurgy, ceramics, glass making, dyes, computers, televisions and other electrical components; they tend to be black or dark brown in color with reddish, yellowish, or more commonly brownish streaks
Halogens _ p5
HALOGENS • Group 17; 7 valence electons • “Halogen" means "salt-former" and compounds containing halogens are called "salts" • Exist in all three states of matter: • Solid- Iodine, Astatine • Liquid- Bromine • Gas- Fluorine, Chlorine • Extremely reactive nonmetals
Noble Gases _ p6
NOBLE GASES • do not form compounds easily because outer shell of electrons is full and does not lose or gain electrons (does not need to bond to be in most stable form) • He: used with O2 for deep sea dives and hot air balloons; Ar and Ne in lights; Kr/Xe in photographic flashes and strobe lamps
Group Numbering 3A 4A 5A 6A 7A 8A 1 2 13 14 15 16 17 18
Electronegativity The ability of an element’s atoms to attract electrons in a chemical bond Generally Increasing Generally Decreases
Electronegativity • Most electronegative element is Fluorine (might want to make a note of that) • Let’s Practice • Between the elements As, Sn and Sb, which is the most electronegative? • As • Between the elements Sr, Ba and Ra, which is the most electronegative? • Sr
Ionization Energy The energy needed to remove an electron from a gaseous atom Generally Increasing Generally Decreases
Ionization Energy • Fluorine has the highest ionization energy of all the elements (might want to make a note) • Let’s Practice Between Na, Mg, K and Ca, which has the highest ionization energy? • Mg • Between Pd, Ag and Cd, which has the highest ionization energy? • Cd
Atomic Radii Half the distance between the nuclei of adjacent, identical atoms in crystals or molecules Generally Decreases Generally Increases
Atomic Radii • Francium has the largest atomic radius (make a note!) • Let’s practice • Of Li, Be, Na and Mg, which has the largest atomic radius? • Na • Of S, Se, Te and Po, which has the largest atomic radius? • Po
More Definitions Physical Property:a property that can be seen or measured without changing the identity of the substance Examples: color, size, density, melting pt Chemical Property: the ability or inability of a substance to react with or change into other substances Examples: flammability, reactivity
Properties • Extensive Property • A physical property that is dependent upon the amount of a substance present. • Ex. Mass, length, volume • Intensive Property • A physical property that remains the same no matter how much of a substance is present. • density