CHEMICAL EQUILIBRIUM 26 JAN 2011
460 likes | 636 Vues
CHEMICAL EQUILIBRIUM 26 JAN 2011. ERT207 ANALYTICAL CHEMISTRY. MISS NOORULNAJWA DIYANA YAACOB. The Concept of Equilibrium. Do all chemical reactions go to completion? When you start with only reactants , do you end up with only products ?

CHEMICAL EQUILIBRIUM 26 JAN 2011
E N D
Presentation Transcript
CHEMICAL EQUILIBRIUM26 JAN 2011 ERT207 ANALYTICAL CHEMISTRY MISS NOORULNAJWA DIYANA YAACOB
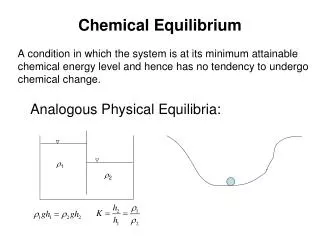
The Concept of Equilibrium Do all chemical reactions go to completion? When you start with only reactants, do you end up with only products? Most of chemical reactions are reversible. They do not go to completion (just products present). In fact, they will be left, at some instance, with a mixture of reactants and products, which are in equilibrium (their concentrations are not changing). At equilibrium the concentrations of the products and the reactants are not changing.
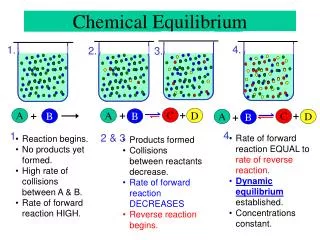
Rate forward Rate reverse Equilibrium, Continued Initially, only the forward reaction takes place. As the forward reaction proceeds it makes products and uses reactants. Because the reactant concentration decreases, the forward reaction slows. As the products accumulate, the reverse reaction speeds up. Once equilibrium is established, the forward and reverse reactions proceed at the same rate, so the concentrations of all materials stay constant. Eventually, the reaction proceeds in the reverse direction as fast as it proceeds in the forward direction. At this time equilibrium is established. Rate Time
Chemical Equilibrium kf kr As time passes, more NO2 is produced and the brown color intensifies. After some time, the brown color stops changing. The concentration of NO2 becomes constant. Same does the concentration of N2O4. At this instance, does the reaction stop? N2O4 (g) 2NO2 (g) Reversible reaction Dr. Al-Saadi
Chemical Equilibrium kf Rate forward = kf[N2O4] Rate backward = kr[NO2]2 kr Reaction progress [N2O4] is high ;[NO2] is zero Rate forward >>Rate backward Rate forward >Rate backward Rate forward =Rate backward Dynamic equilibrium is established The reaction does not stop. It continues running on both directions at equal rates. N2O4 (g) 2NO2 (g) The reaction is reversible, i.e. it does NOT stop but proceeds at both directions. Dr. Al-Saadi
Chemical Equilibrium kf Rate forward = kf[N2O4] Rate backward = kr[NO2]2 kr The rates of the reaction (forward and backward rates) change over time. The forward rate gradually decreases, while the backward rate gradually increases (starting from zero). Eventually, at equilibrium, both rates become equal. N2O4 (g) 2NO2 (g)
At equilibrium the rates of the forward and reverse reactions are equal, and the concentrations of the reactants and products are constant.
The equilibrium constant (Keq) is a value representing the unchanging concentrations of the reactants and the products in a chemical reaction at equilibrium.
aA + bB cC + dD → → For the general reaction at a given temperature
3H2 + N2 2NH3 → → For the reaction
4NH3 + 3O2 2N2+ 6H2O → → For the reaction
The magnitude of an equilibrium constant indicates the extent to which the forward and reverse reactions take place.The larger the equilibrium constant, the farther to the right is the reaction at equilibrium. H2 + I2 2HI At equilibrium more product than reactant exists. At equilibrium more reactant than product exists. COCl2 CO+ Cl2 → → → →
Magnitude of Equilibrium Constant ( ) a A + b B c C + d D eq The value of Kc is very large [C]c[D]d Kc = [A]a[B]b The value of Kc is very small The value of Kc is neither large nor small • For the above reaction, three outcomes are possible: • The reaction goes to completion. The equilibrium mixture will consist predominantly from products. • The reaction doesn't occur to any significant degree. The equilibrium mixture will consist predominantly from reactants. • The reaction occurs to a significant degree, but not to completion. The equilibrium mixture will have both reactants and products in comparable quantities.
Magnitude of Equilibrium Constant ( ) a A + b B c C + d D eq Kc [C]c[D]d Kc = 10-2 102 [A]a[B]b The magnitude of Kc is very small. The reaction will almost not occur The reaction will have an equilibrium mixture of both reactants and products. The magnitude of Kc is very large. The reaction will go almost to completion
TYPES OF EQUILIBRIA • We can write equilibrium constants for many types of chemical processes. The equilibria may represent : • dissociation (acid/base, solubility) • Formation of products (complexes) • Reaction (redox) • Distribution between 2 phases (water and nonaqueous solvent
Le Châtelier's Principle Le Châtelier's principle states that: If a change is imposed on a system at equilibrium, the system will respond by shifting in the (forward or reverse) direction that minimizes the effect of that change. As a result, an new equilibrium position will be reestablished. .
equilibrium non-equilibrium Le Châtelier’s Principle When a change is imposed on a system at equilibrium, the system will react in the direction that reduces the amount of change
Changes made on the system can be: • Addition or removal of a reactant or product (Effect of concentration) • change in the volume and pressure of the system (Effect of pressure). • change in temperature (Effect of temperature). Dr. Al-Saadi
For most reactions the rate of reaction increases as reactant concentrations increase. An equilibrium is disturbed when the concentration of one or more of its components is changed. As a result, the concentration of all species will change and a new equilibrium mixture will be established
Changes in Concentration ChangeShift in Equilibrium Increase in [Products] left Decrease in [Products] right Increase in [Reactants] right Decrease in [Reactants] left
Equilibrium shifts to left decrease Cl2 concentration decrease H3O+ concentration increase H2O concentration increase Cl- concentration increase HOCl concentration Equilibrium shifts to left Cl2(aq) +2H2O(l) HOCl(aq) + H3O+(aq) + Cl-(aq) → Equilibrium shifts to left Equilibrium shifts to right Equilibrium shifts to right → Effect of Concentration Changeson the Chlorine Water Equilibrium
Changes in pressure significantly affect the reaction rate only when one or more of the reactants or products is a gas and the reaction is run in a closed container. The effect of increasing the pressure is to increase the concentrations of any gaseous reactants or products
Changes in Pressure and Volume ChangeShift in Equilibrium Increase in Pressure Side with fewest moles Decrease in Pressure Side with most moles Increase in Volume Side with most moles Decrease in Volume Side with fewest moles
In a system composed entirely of gases, a increase in the pressure of the container will cause the reaction and the equilibrium to shift to the side that contains the smallest number of molecules.
N2(g) + 3H2(g) 2NH3(g) 1 mol 3 mol 2 mol 6.02 x 1023 molecules 1.81 x 1024 molecules 1.20 x 1024 molecules 2.41 x 1024 molecules → → Equilibrium shifts to the right towards fewer molecules. Increase Pressure
N2(g) + O2(g) 2NO(g) 1 mol 1 mol 2 mol 6.02 x 1023 molecules 6.02 x 1023 molecules 1.20 x 1024 molecules 1.20 x 1024 molecules → → Increase Pressure Equilibrium does not shift. The number of molecules is the same on both sides of the equation.
Change in Volume and Pressure • Generally, • A decrease in the volume of a reaction vessel will cause a shift in the equilibrium in the direction that minimizes the total number of moles. • An increase in the volume of a reaction vessel will cause a shift in the equilibrium in the direction that maximizes the total number of moles.
Change in Volume and Pressure • Generally, • A decrease in the volume of a reaction vessel will cause a shift in the equilibrium in the direction that minimizes the total number of moles. • An increase in the volume of a reaction vessel will cause a shift in the equilibrium in the direction that maximizes the total number of moles.
When the temperature of a system is raised, the rate of reaction increases. The rate of the reaction that absorbs heat is increased to a greater extent, and the equilibrium shifts to favor that reaction. When the process is endothermic, the forward (left to right) reaction is increased. When the process is exothermic, the reverse (right to left) process is increased. In a reversible reaction, the rates of both the forward and the reverse reactions are increased by an increase in temperature.
At 1000oC moles CO2moles CO C(s) + CO2(g) + heat 2CO(g) → Equilibrium shifts to right → Heat may be treated as a reactant in endothermic reactions. At room temperature very little CO forms.
Change in Temperature Unlike the case with concentration and pressure changes, the change in temperature of a chemical reaction can change the value of the equilibrium constant. It makes the reaction faster or slower, depending on the enthalpy change (ΔH) “heat” accompanying the reaction.
Change in Temperature • N2O4(g) 2NO2(g) ΔH= 58.0 kJ/mol • Let’s apply here Le Châtelier's principle to the heat absorbed as a reactant. Heat + N2O4(g) 2NO2(g) Adding heat means the reaction will be shifted to the right. Also, addition of heat means an increase in temperature. • In general, increasing the temperature of endothermic reactions shifts it to the right. While decreasing the temperature of endothermic reactions shifts it to the left.
Change in Temperature yellow brown yellow Heating ? brown N2O4(g) 2NO2(g) ΔH= 58.0 kJ/mol
Change in Temperature blue pink Heating Cooling CoCl42- and Co(H2O)62+ ions at equilibrium Consider the following exothermic reaction: CoCl42- + 6H2O Co(H2O)62+ + 4Cl- + Heat
Change in Temperature The increase in temperature favors endothermic reactions. The decrease in temperature favors exothermic reactions. The change in temperature not only affects the equilibrium position, but also alters the value of the equilibrium constant.
Changes in Temperature ChangeEndo. RxExo. Rx Increase T K decreases K increases Decrease T K increases K decreases
A catalyst is a substance that influences the rate of a reaction and can be recovered essentially unchanged at the end of the reaction. A catalyst does not shift the equilibrium of a reaction. It affects only the speed at which the equilibrium is reached.
Energy Diagram for an Exothermic Reaction Activation energy: the minimum energy required for a reaction to occur. A catalyst speeds up a reaction by lowering the activation energy. A catalyst does not change the energy of a reaction.
2KClO3(s) → 2KCl + 3O2(l) The laboratory preparation of oxygen uses manganese dioxide as a catalyst to increase the rate of the reaction. AlCl3 PCl3(l)+ S(s) → PSCl3(l) Very little thiophosphoryl chloride is formed in the absence of a catalyst because the reaction is so slow. In the presence of a catalyst the reaction is complete in a few seconds. MnO2 Δ
2KClO3(s) → 2KCl + 3O2(l) The laboratory preparation of oxygen uses manganese dioxide as a catalyst to increase the rate of the reaction. AlCl3 PCl3(l)+ S(s) → PSCl3(l) Very little thiophosphoryl chloride is formed in the absence of a catalyst because the reaction is so slow. In the presence of a catalyst the reaction is complete in a few seconds. MnO2 Δ