Types of Bonding Water Molecule Formation & Configuration Unusual Properties Hydrogen Bonding Heat Capacity Phases
640 likes | 816 Vues
S. Types of Bonding Water Molecule Formation & Configuration Unusual Properties Hydrogen Bonding Heat Capacity Phases of Water Adding Salts to Water Constituents of Seawater Sampling Devices Effects of Density and Salinity Hydrologic Cycle Composition of River Water Residence Times

Types of Bonding Water Molecule Formation & Configuration Unusual Properties Hydrogen Bonding Heat Capacity Phases
E N D
Presentation Transcript
S Types of Bonding Water Molecule Formation & Configuration Unusual Properties Hydrogen Bonding Heat Capacity Phases of Water Adding Salts to Water Constituents of Seawater Sampling Devices Effects of Density and Salinity Hydrologic Cycle Composition of River Water Residence Times Dissolved Gases pH of Seawater
P Covalent Bonding
P Ionic Bonding
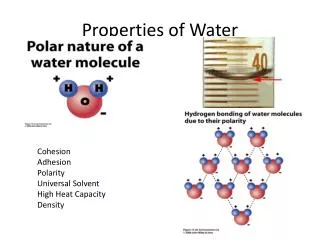
S WATER’S UNUSUAL PROPERTIES
P Hydrogen bonding • Polarity means small negative charge at O end • Small positive charge at H end • Attraction between + and – ends of water molecules to each other or other ions Fig. 5.3
P Hydrogen bonding • Hydrogen bonds are weaker than covalent bonds but still strong enough to result in: • High surface tension • High solubility of chemical compounds in water • Solid, liquid, gas at Earth’s surface • Unusual thermal properties • Unusual density
P CALORIE the amount of energy required to raise the temperature of 1 gm of liquid water 1 degree Centigrade
P HEAT CAPACITY (SPECIFIC HEAT) The amount of energy required to raise the temperature of 1 gm of a substance 1 degree Centigrade
What does this mean? • If we had 3 cookie sheets each 1 cm deep • One filled with water, one with soil and one with sand • The amount of incoming energy that would heat the water 1oC, would heat the soil 1.4oC and the sand 5oC
P Unusual thermal properties of H2O • Water - high heat capacity • Amount of heat required to raise the temperature of 1 gram of any substance 1o C • Water can take in/lose lots of heat without changing temperature very much • Rocks - low heat capacity • Rocks quickly change temperature as they gain/lose heat
S 501 Day/Night Temperature Differences Large on land, small in the ocean
S Global thermostatic effects • Moderate temperature on Earth’s surface • Equatorial oceans (hot) don’t boil • Polar oceans (cold) don’t freeze solid • Marine effect • Oceans moderate temperature changes day/night; different seasons • Continental effect • Land areas have greater range of temperatures day/night and during different seasons
Unusual thermal properties of H2O • H2O has high boiling point • H2O has high freezing point • Most H2O is in the form of water (liquid) on Earth’s surface (good for life) • High latent (hidden) heats of • Vaporization/condensation • Melting/freezing • Evaporation
P Specific Heat = 1.0 calories/ gm Specific Heat = 0.5 calories/gm Fig. 5.6
P Water molecules in different states of matter Fig. 5.5
S Show water phase animation
P Changes of state due to adding or subtracting heat • Heat is energy of moving molecules • Temperature is measurement of average kinetic energy
S Elements within columns share similar properties
S Ionic bonding, loosely held together
P Dipolar water molecules break ionic bonds by surrounding sodium and chloride ions
S CONSTITUENTS OF SEAWATER
Black Sand Sugar Black Sand Clear
P Salinity • Total amount of solid material dissolved in water • Typical salinity is 3.5% or 35o/oo • Six elements make up 99% of dissolved solids in seawater Fig. 5.12
P SALINITY UNITS % = PERCENT OR PARTS PER HUNDRED (PPH) Since open ocean salinity varies from 3.3-3.7%, we move the decimal one place to the right and express it as 0/00 OT PARTS PER THOUSAND (PPT) 3.3-3.7% BECOMES 33-37 o/oo
P Measuring salinity • Evaporation • Chemical analysis • Principle of constant proportions • Major dissolved constituents in same proportion regardless of total salinity • Measure amount of chlorine (chlorinity) • Electrical conductivity • Salinometer • CTD
S Salinity variations • Open ocean salinity 33 to 37 o/oo • Coastal areas salinity varies more widely • Influx of freshwater lowers salinity or creates brackish conditions • Greater rate of evaporation raises salinity or creates hypersaline conditions • Salinity may vary with seasons (dry/rain)
9/23 P > 100 ppm >1, <100 ppm < 1 ppm
P Forchammer’s Principle (Rule of Constant Proportions) 2/12 Although the salinity of seawater may change from place to place, the ratio of ions to each other remains constant Importance: You only need to measure one ion to calculate the concentration of others - this can be the cheapest or easiest one to measure
P How to change salinity • Add water • Remove water • Add dissolved substances • Remove dissolved substances
S Precipitation (rain or snow) Runoff (river flow) Melting icebergs Melting sea ice Evaporation Formation of sea ice Processes that add/subtract water from oceans Salinity increases through: Salinity decreases through:
S Tourists watching sea ice form
S Nansen bottle
S Water-sampling bottles
S Salinity is most commonly measured by electrical conductivity
P Density of water • Density of water increases as temperature decreases down to 4oC • From 4oC to 0oC density of water decreases as temperature decreases • Density of ice is less than density of water
P Density of water Fig. 5.10
S Slides 46-58 Density effects in freshwater