Compounds of Carbon
370 likes | 1.21k Vues
Compounds of Carbon. Carbon has a variable valency of 2 and 4 two important kinds of oxides namely carbon monoxide and carbon dioxide. Both these oxides are gases at room temperature. . Carbon Dioxide - Occurrence .

Compounds of Carbon
E N D
Presentation Transcript
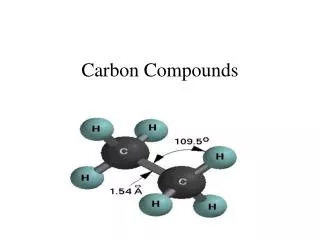
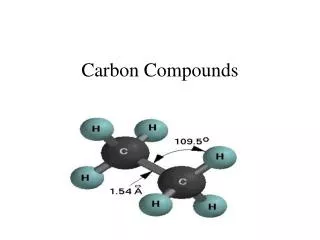
Carbon has a variable valency of 2 and 4 • two important kinds of oxides namely carbon monoxide and carbon dioxide. • Both these oxides are gases at room temperature.
Carbon Dioxide - Occurrence • an Baptista van Helmont discovered the presence of carbon dioxide in the year 1630. • It is added to the air by the following processes: a) Respiration of all animals and plants b) Combustion of carbonaceous fuels c) Decay of organic matter d) Fermentation e) As a result of volcanic eruptions. In the form of compounds, it is present as carbonate and bicarbonate salts.
Carbon cycleThe cyclic changes that carbon undergoes in nature is referred to as the Carbon cycle.
on burning wood, the main products obtained are carbon dioxide and water vapours.
Remember : • Dilute sulphuric acid should not be used in this preparation as it forms a white insoluble layer of calcium sulphate around the marble chips. This coating does not allow the marble chips to come in contact with the acid. As result, the reaction stops.
Tests for Carbon Dioxide • Carbon dioxide is a colourless and almost tasteless gas that puts off a flame. • It turns blue litmus paper red. • turns limewater milky. • In carbon dioxide, a burning magnesium ribbon continues to burn .
Physical Properties of Carbon Dioxide • Carbon dioxide is a colourless, odourless gas very slightly sour in taste. • Density :carbon dioxide is heavier than air. Its vapour density is 22 (Vapour density of air = 14.4). • Solubility:Itis only slightly soluble in water. • Liquefaction:Whencompressed to 70 atmospheres at room temperature, it liquefies to form liquid carbon dioxide. The liquid carbon dioxide solidifies to form a snow like solid carbon dioxide, commonly called 'Dry ice'.
Chemical Properties of Carbon Dioxide • Carbon dioxide is slightly acidic. It turns blue litmus paper red. • Stability:Itis very stable gas at ordinary temperature and pressure. • Combustibility • Carbon dioxide is neither combustible, not a supporter of combustion. A burning splinter or a burning candle, gets put off , but metals like potassium, sodium, magnesium etc. continue to burn in carbon dioxide.
Uses of Carbon Dioxide • Carbon dioxide is used in photosynthesis by green plants to produce carbohydrates. • To induce natural breathing. • To extinguish fires • As a refrigerant:Solidcarbon dioxide called "Dry ice" can provide temperatures as low as -109.3o F. It is superior to ordinary ice, for the following reasons
In medicine A mixture of 97% oxygen and 3% carbon dioxide, called carbogen is used to revive persons affected by carbon monoxide poisoning, pneumonia, asphyxiation etc. • Manufacture of aerated drinks • Food storage • In the entertainment industry