Fossil Fuel Overview
260 likes | 454 Vues
Fossil Fuel Overview. GLY 4241 - Lecture 14 Fall, 2014. Fossil Fuel. Fossil fuels are fuels formed from once living organisms - they include 1. Petroleum 2. Natural gas 3. Coal 4. Fuels derived from oil shale and tar shale. Fossil Fuel Chemistry.

Fossil Fuel Overview
E N D
Presentation Transcript
Fossil Fuel Overview GLY 4241 - Lecture 14 Fall, 2014
Fossil Fuel • Fossil fuels are fuels formed from once living organisms - they include • 1. Petroleum • 2. Natural gas • 3. Coal • 4. Fuels derived from oil shale and tar shale
Fossil Fuel Chemistry • All fossil fuels are carbon based – combustion of these fuels produces carbon dioxide, a GHG, and other substances • Natural gas, petroleum, and fuels derived from oil shale and tar shale are hydrocarbons - combustion of these fuels produces carbon dioxide, and water vapor, as well as other substances
“Fossil” • As a noun, fossil refers to a relic, remnant, or representation of an organism that existed in a past geological age, or of the activity of such an organism, occurring in the form of mineralized bones, shells, etc., as casts, impressions, and molds, or as frozen preserved organisms • As an adjective, it means having the characteristics or nature of a fossil
Formation of Fossil Fuels • In order to form fossil fuels, large accumulations of organic matter must be quickly buried, to prevent rapid decomposition into carbon dioxide and water • Coal forms in fresh-water environments, and forms from terrestrial plant matter • Petroleum, liquid organic matter, is typically formed in salt-water environments, from marine microorganisms
Relation to Hypoxia • Fossil fuels must be protected from rapid decomposition • Deposition into hypoxic to anoxic environments will greatly slow the decomposition processes • If oxidation is possible, it yields heat, which increases the rate of breakdown
Coal Formation • When subjected to heat and pressure by burial, the plant hydrocarbon decomposes, to yield a materiel richer and richer in carbon • This corresponds to an increase in coal rank
Coal Rank • Coal is divided into ranks • Peat • Lignite • Sub-bituminous • Bituminous • Anthracite
Coal “Coking” • Many industrial uses, including the smelting of iron, require a fuel that is free of volatiles and ash • Coal is heated in the absence of oxygen • Volatile hydrocarbons such as propane, benzene and other aromatic hydrocarbons, some sulfur gases, and water are driven off • This leaves coke, a hard, gray, porous material
Anthracite • Anthracite is actually a metamorphic rock
Fossil Fuel Formation Video • Interview with Professor Cairncross, of the University of Johannesburg
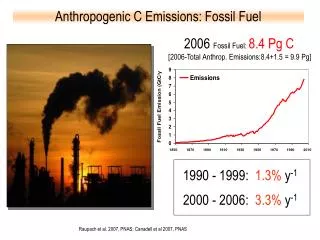
Greenhouse Gas Effects • All fossil fuels contribute to the Greenhouse effect • When hydrocarbons are burned, they produce both carbon dioxide and water • Both are GHG, but water vapor reaches saturation in the atmosphere and precipitates • Carbon dioxide remains longer in the atmosphere
Comparison of Fossil Fuels • Natural gas is methane, CH4, which means 4 molecules of water and one of carbon dioxide per molecule methane • For petroleum, the ratio varies, but is around 2 H per carbon • For coal, as grade increase, it is almost pure carbon
Carbon Content of Coal • Lignite 25-35% C • Bituminous 60-80% C • Anthracite 92-98% C
Environmental Effects of Coal • Thus, as far as greenhouse effect is concerned, coal is the worst fuel • Coal also produces the most pollutants • This includes mercury • Coal often releases uranium, and produces radioactive emissions • Coal also produces sulfur gases, but these may be removed by power plant scrubbers
Or Is It? • Natural-gas operations in areas such as Wyoming’s Jonah Field could release far more methane into the atmosphere than previously thought.
Pétron et al. Study (2014) • Based on airplane sampling of the Colorado Denver-Julesburg Basin on two days in May, 2012, they found estimated methane emissions of 19.3 ± 6.9 tons/hour • This is 3x higher than EPA’s Greenhouse Gas reporting Data estimates
Pétron et al. Comment • “Assuming that these emissions are solely originating from O&G-related activities in the study region, our results show that the state inventory for total volatile organic compounds emitted by O&G activities is at least a factor of 2 too low for May 2012.”
Benzene Emissions • Pétron et al. found that benzene emissions from oil and gas operations were 173 ± 64 kg/h, seven times higher than the state inventory indicates • Benzene is the most soluble aromatic compound, and is a known carcinogen
Methane Emissions • Methane emissions are a large concern for climate change • Carbon dioxide equivalency is a quantity that describes, for a given mixture and amount of greenhouse gas, the amount of CO2 that would have the same global warming potential (GWP), when measured over a specified timescale (generally, 100 years) • For methane, the GWP has usually been quoted in the range 21-25 for the 100 year period • IPCC raised the 100 year figure to 28 in its 2013 AR5 report • From AR5 (2013), IPCC gave a GWP for methane of 84 over a 20 year frame of reference
Consequences of Methane Leaks • This means that methane is far more dangerous than carbon dioxide in the shorter term • Over the longer term, it oxidizes to produce carbon dioxide, which must then be removed from the atmosphere • Failure to account for increased rates of methane emissions from anthropogenic sources, which also include methane degassing from permafrost melting, is another reason to doubt IPCC projections for both temperature increases and sea-level rise