Titrations
470 likes | 943 Vues
Neutralization Reactions. Titrations. Standard Solutions: strong acids or strong bases because they will react completely Acids: ( HCl ), ( HClO 4 ), ( H 2 SO 4 ) Bases: ( NaOH ), ( KOH ). Acid/Base Indicators

Titrations
E N D
Presentation Transcript
Neutralization Reactions Titrations http:\\asadipour.kmu.ac.ir 48slides
Standard Solutions: strong acids or strong bases because they will react completely • Acids: (HCl), (HClO4), (H2SO4) • Bases: (NaOH), (KOH) http:\\asadipour.kmu.ac.ir 48slides
Acid/Base Indicators Many substances display colors that depend on the pH of the solutions in which they are dissolved. An acid/base indicator is a weak organic acid or a weak organic base whose undissociated form differs in color from its conjugate form. e.g., the behavior of an acid-type indicator, HIn, is described by the equilibrium HIn+ H2O In- + H3O+ acid color base color The equilibrium for a base-type indicator, In, is In + H2O InH+ + OH- base color acid color http:\\asadipour.kmu.ac.ir 48slides
…continued… The equilibrium-constant expression for the dissociation of an acid-type indicator takes the form Rearranging leads to The hydronium ion concentration determines the ratio of the acid to the conjugate base form of the indicator and thus determines the color developed by the solution. - + + - http:\\asadipour.kmu.ac.ir 48slides
…continued… The color imparted to a solution by a typical indicator appears to the average observer to change rapidly only within the limited concentration ratio of approximately 10 to 0.1 and its base color when The color appears to be intermediate for ratios between these two values. These ratios vary considerably from indicator to indicator. http:\\asadipour.kmu.ac.ir 48slides
…continued… For the full acid color, [H3O+] = 10Ka and similarly for the full base color, [H3O+] = 0.1Ka To obtain the indicator pH range, we take the negative logarithms of the two expression: pH (acid color) = -log (10Ka) = pKa-1 pH (basic color) = -log (0.1Ka) = pKa+1 indicator pH range = pKa 1 http:\\asadipour.kmu.ac.ir 48slides
Variables: 1)temperature,2)ionic strength of medium 3)presence of organic solvents 4)presence of colloidal particles http:\\asadipour.kmu.ac.ir 48slides
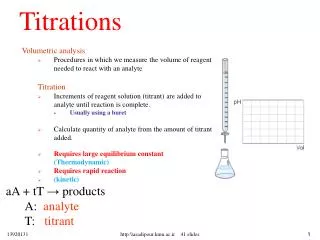
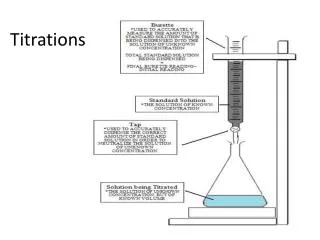
Neutralization Reactions • Neutralization is the reaction of an acid and a base. • Titration is a common technique for conducting a neutralization. • At the equivalence point in a titration, the acid and base have been brought together in exact stoichiometric proportions. • The point in the titration at which the indicator changes color is called the end point. • The indicator endpoint and the equivalence point for a neutralization reaction can be best matched by plotting a titration curve, a graph of pH versus volume of titrant. • In a typical titration, 50 mL or less of titrant that is 1 M or less is used. http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve ForStrong Acid - Strong Base HCl + NaOH→ NaCl +H2O Calculate the pH at the some points and draw the curve. 4 essential points. 1)initial point 2)equivalence point 3)before the equivalence point 4)beyond the equivalence point http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve ForStrong Acid - Strong Base HCl + NaOH→ NaCl +H2O 4 questions. 1)What are the present compounds? 2)Which of them is effective on pH? 3)How much are the concentrations? 4)What is the relationship between their Conc. And pH? http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve ForStrong Acid - Strong Base HCl + NaOH→ NaCl +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M HCl with 0.500 M NaOH. • initial pH. (Before the addition of any NaOH) . Answer Q1. There are:HCl & H2O Answer Q2.HCl Answer Q3. [HCl] Answer Q4. pH=-log[H+] http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve ForStrong Acid - Strong Base HCl + NaOH→ NaCl +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M HCl with 0.500 M NaOH. b)equivalence point. Answer Q1. There are:NaCl & H2O Answer Q2. H2O Answer Q3. Answer Q4. pH=7 http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve ForStrong Acid - Strong Base HCl + NaOH→ NaCl +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M HCl with 0.500 M NaOH. c)before the equivalence point. Answer Q1. There are:HCl,NaCl & H2O Answer Q2.HCl Answer Q3. Answer Q4. [H+]=NpH=-log[H+] http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve ForStrong Acid - Strong Base HCl + NaOH→ NaCl +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M HCl with 0.500 M NaOH. d)after the equivalence point. Answer Q1. There are:NaOH,NaCl & H2O Answer Q2.NaOH Answer Q3. Answer Q4. [OH-]=N pOH=-log[OH-] pH=14-pOH http"\\asadipour.kmu.ac.ir........52 slides
Titration Curve ForStrong Acid - Strong Base pH is low at the beginning. pH changes slowly until just before equivalence point. pH changes sharply around equivalence point. pH = 7.0 at equivalence point. Further beyond equivalence point, pH changes slowly. Any indicator whose color changes in pH range of 4 – 10 can be used in titration. http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve Forweak acid- Strong Base CH3COOH + NaOH→ CH3COO- + Na+ +H2O Calculate the pH at the some points and draw the curve. Ka=1×10-5 5 essential points. 1)initial point 2)equivalence point 3)beyond the initial point 4)before the equivalence point 5)beyond the equivalence point http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve Forweak acid- Strong Base CH3COOH + NaOH→ CH3COO- + Na+ +H2O 4 questions. 1)What are the present compounds? 2)Which of them is effective on pH? 3)How much are the concentrations? 4)What is the relationship between their Conc. And pH? http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve Forweak acid- Strong Base CH3COOH + NaOH→ CH3COO- + Na+ +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M CH3COOH with 0.500 M NaOH. • initial pH. (Before the addition of any NaOH) . Answer Q1. There are: CH3COOH & H2O Answer Q2. CH3OOH Answer Q3. CH3OOH Answer Q4. pH=-log[H+] http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve Forweak acid- Strong Base CH3COOH + NaOH→ CH3COO- + Na+ +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M CH3COOH with 0.500 M NaOH. b)equivalence point. Answer Q1. There are:CH3COO- , Na+ & H2O Answer Q2.CH3COO- Answer Q3. Answer Q4. pOH=-log[OH-] Ka×Kb=Kw http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve Forweak acid- Strong Base CH3COOH + NaOH→ CH3COO- + Na+ +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M CH3COOH with 0.500 M NaOH. c)beyond the initial point. Answer Q1. There are: CH3COOH,CH3COO- ,Na+ & H2O Answer Q2. CH3COOH,CH3COO- Answer Q3. Answer Q4. http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve Forweak acid- Strong Base CH3COOH + NaOH→ CH3COO- + Na+ +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M CH3COOH with 0.500 M NaOH. d)before the equivalence point. Answer Q1. There are: CH3COOH,CH3COO- ,Na+ & H2O Answer Q2. CH3COOH,CH3COO- Answer Q3. Answer Q4. http"\\asadipour.kmu.ac.ir........52 slides
Drawing titration Curve Forweak acid- Strong Base CH3COOH + NaOH→ CH3COO- + Na+ +H2O Calculate the pH at the following points in the titration of 20.00 mL of 0.500 M CH3COOH with 0.500 M NaOH. e)after the equivalence point. Answer Q1. There are:NaOH,CH3COO- , Na+ & H2O Answer Q2.NaOH Answer Q3. Answer Q4. [OH-]=N pOH=-log[OH-] pH=14-pOH http"\\asadipour.kmu.ac.ir........52 slides
Titration Curve ForWeak Acid - Strong Base The initial pH is higher because weak acid is partially ionized. At the half-neutralization point, pH = pKa. pH >7 at equivalence point because the anion of the weak acid hydrolyzes. The steep portion of titration curve around equivalence point has a smaller pH range. The choice of indicators for the titration is more limited. http"\\asadipour.kmu.ac.ir........52 slides
Titration curves for HCl with NaOH. A. 50.0 ml of 0.0500 M HCl with 0.1000M NaOH. B. 50.00 ml of 0.000500 M HCl with 0.00100 M NaOH. http:\\asadipour.kmu.ac.ir 48slides
Titration curves for the titration of acetic acid with NaOH. A. 0.1000 M acetic acid with 0.1000M NaOH. B. 0.001000 M acetic acid with 0.00100 M NaOH. http:\\asadipour.kmu.ac.ir 48slides
Titration curves for the titration of acetic acid with NaOH. A. 0.1000 M acetic acid with 0.1000M NaOH. B. 0.001000 M acetic acid with 0.00100 M NaOH. Titration curves for HCl with NaOH. A. 50.0 ml of 0.0500 M HCl with 0.1000M NaOH. B. 50.00 ml of 0.000500 M HCl with 0.00100 M NaOH. http:\\asadipour.kmu.ac.ir 48slides
General Shapes of Titration Curves Effect of pKa Effect of initial concentration http:\\asadipour.kmu.ac.ir 48slides
Effect of Ka The effect of acid strength (dissociation constant) on titration curves. Each curve represents the titration of 50.00 ml of 0.1000 M acid with 0.1000 M base. http:\\asadipour.kmu.ac.ir 48slides
Effect of Kb The effect of base strength (dissociation constant) on titration curves. Each curve represents the titration of 50.00 ml of 0.1000 M base with 0.1000 M HCl. http:\\asadipour.kmu.ac.ir 48slides
non-aqueous acid base titration 1)Solubility 2) acid or base strength Acid and base strengths that are not distinguished in aqueous solution may be distinguishable in non-aqueous solvents. HClO4 > HClin acetic acid solvent, neither acid is completely dissociated. HClO4 + CH3COOH ClO4– + CH3COOH2+K = 1.3×10–5 strong acid strong base weak base weak acid HCl + CH3COOH Cl– + CH3COOH2+K = 5.8×10–8 Differentiate acidity or basicity of different acids or bases differentiating solvent for acids …… acetic acid differentiating sovent for bases …… ammonia http:\\asadipour.kmu.ac.ir 48slides
Reaction between weak acid and weak base • Both the weak acid and weak base remain largely undissociated and neutralization involves proton transfer from the weak acid to the weak base. Consider acetic acid and ammonia: • CH3COOH + NH3 CH3COO- + NH4+ is composed of • CH3COOH + H2O CH3COO- + H3O+ K1 = Ka = 1.8 x 10-5 • NH3 + H2O NH4+ + OH- K2 = Kb = 1.8 x 10-5 • H3O+ + OH-2 H2O K3 = 1/ Kw = 1 x 1014 Kn = Koverall = K1 x K2 x K3 = Kb Ka / Kw = 3.2 x 104 • Therefore, the Reaction Still Shifts significantly to the right -- ionizing much of the component present in the smaller amount http:\\asadipour.kmu.ac.ir 48slides
Titration problems • What volume of 0.10 mol/L NaOH is needed to neutralize 25.0 mL of 0.15 mol/L H3PO4? • 25.0 mL of HCl(aq) was neutralized by 40.0 mL of 0.10 mol/L Ca(OH)2 solution. What was the concentration of HCl? • A truck carrying sulfuric acid is in an accident. A laboratory analyzes a sample of the spilled acid and finds that 20 mL of acid is neutral-ized by 60 mL of 4.0 mol/L NaOH solution. What is the concentration of the acid? • What volume of 1.50 mol/L H2S will neutral-ize a solution containing 32.0 g NaOH? http:\\asadipour.kmu.ac.ir 48slides
Titration problems 1. (3)(0.15 M)(0.0250 L) = (1)(0.10 M)(VB) VB= (3)(0.15 M)(0.0250 L) / (1)(0.10 M) = 0.11 L 2. (1)(MA)(0.0250 L) = (2)(0.10 M)(0.040 L) MA= (2)(0.10 M)(0.040 L) / (1)(0.0250 L) = 0.32 M 3. Sulfuric acid = H2SO4 (2)(MA)(0.020 L) = (1)(4.0 mol/L)(0.060 L) MA = (1)(4.0 M)(0.060 L) / (2)(0.020 L) = 6.0 M 4. mol NaOH = 32.0 g x 1 mol/40.00 g = 0.800 (2)(1.50 mol/L)(VA) = (1)(0.800 mol) VA= (1)(0.800 mol) / (2)(1.50 mol/L) = 0.267 L http:\\asadipour.kmu.ac.ir 48slides
Species concentrations of weak diprotic acids Evaluate concentrations of species in a 0.10 M H2S solution. Solution: H2S = H+ + HS–Ka1 = 1.02e-7 (0.10–x) x+y x-y Assume x = [HS–] HS– = H+ + S2–Ka2 = 1.0e-13 x–y x+y y Assume y = [S2–] (x+y) (x-y) (x+y) y ————— = 1.02e-7 ———— = 1.0e-13 (0.10-x) (x-y) [H2S] = 0.10 – x = 0.10 M[HS–] = [H+] = x y = 1.0e–4 M; [S2–] = y = 1.0e-13 M 0.1>> x >> y: x+ y = x-y = xx = 0.1*1.02e-7 = 1.00e-4y = 1e-13 http:\\asadipour.kmu.ac.ir 48slides
Alpha Values • Def.: the relative equilibrium concentration of the weak acid/base and its conjugate base/acid • (titrating HOAcwith NaOH): α0+ α1 = 1 http:\\asadipour.kmu.ac.ir 48slides
Plots of relative amounts of acetic acid and acetate ion during a titration. http:\\asadipour.kmu.ac.ir 48slides