Classification of Matter
420 likes | 800 Vues
Classification of Matter. Chapter 1 Chemistry 2-a. Chemistry : the field of study concerned with the characteristics, composition, and transformations of matter Matter : anything that has mass and occupies space Living and non-living Macroscopic and microscopic. States of Matter. Solids.

Classification of Matter
E N D
Presentation Transcript
Classification of Matter Chapter 1 Chemistry 2-a
Chemistry: the field of study concerned with the characteristics, composition, and transformations of matter • Matter: anything that has mass and occupies space • Living and non-living • Macroscopic and microscopic
Solids • Definite shape • Definite volume • Atoms packed tightly together • May be crystalline or amorphous • Very low compressibility
Solids • Some solids have their particles arranged in an orderly geometric pattern—we call these crystalline solids. • Salt and diamonds. • Other solids have particles that do not show a regular geometric pattern over a long range—we call these amorphous solids. • Plastic and glass. Tro's "Introductory Chemistry", Chapter 3
Liquids • No definite shape • Definite volume • Atoms close together, but not locked into place • Low compressibility
Gases • No definite shape • No definite volume • Atoms/Molecules far apart • Compressible
Gases • Because there is a lot of empty space, the particles can be squeezed closer together. Therefore, gases are compressible. • Because the particles are not held in close contact and are moving freely, gases expand to fill and take the shape of their container, and will flow. Tro's "Introductory Chemistry", Chapter 3
Classifying Matterby Physical State • Matter can be classified as solid, liquid, or gas based on what properties it exhibits. • Fixed = Property doesn’t change when placed in a container. • Indefinite = Takes the property of the container. Tro's "Introductory Chemistry", Chapter 3
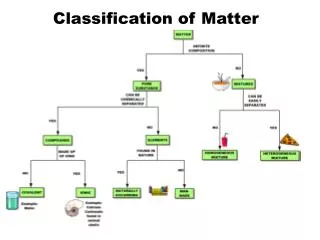
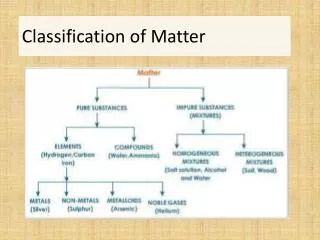
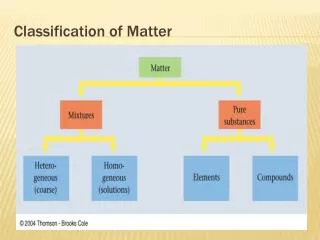
Classification of Matter1. Pure Substances Element: a substance that cannot be decomposed or transformed into other chemical substances by ordinary chemical processes Atom: smallest particle of an element that can exist and still have the properties of that element Ex: Aluminum (Al), Carbon (C), Neon (Ne), Potassium (K)
Copper—A Pure Substance • Color is brownish red. • Shiny, malleable, and ductile. • Excellent conductor of heat and electricity. • Melting point = 1084.62 °C • Density = 8.96 g/cm3 at 20 °C Tro's "Introductory Chemistry", Chapter 3
Compound: A substance consisting of two or more different atoms chemically bonded in a fixed ratio • NaCl, CO2 • Ionic compounds and molecules
Classification of Pure Substances Elements Compounds 1. Made of one type of atom. (Some elements are found as multi-atom molecules in nature.) 2. Combine together to make compounds. 1. Made of one type of molecule, or array of ions. 2. Molecules contain 2 or more different kinds of atoms. Tro's "Introductory Chemistry", Chapter 3
Practice—Classify the Following as Elements or Compounds, Continued • Chlorine, Cl2 = element. • Table sugar, C12H22O11 = compound. • A red solid that turns blue when heated and releases water that is always 30% of the solid’s mass = compound. • A brown-red liquid that, when energy is applied to it in any form, causes only physical changes in the material, not chemical = element. Tro's "Introductory Chemistry", Chapter 3
2. Mixtures Mixtures: combinations of two or more pure substances in which each substance retains its own identity • Heterogeneous Mixture: a substance in which elements and/or compounds are blended together in such a way that there is no uniform composition or fixed ratio of the components of the mixture • Examples: Oil and water, mixed nuts
Homogeneous Mixture: A substance in which the different elements/compounds being mixed exist in definite ratios, but are not chemically bonded • Consists of two or more substances in the same phase • No amount of magnification will reveal an interface • Called a “solution” • Examples: Salt water, sugar water, O2 dissolved in water
Practice—Classify the Following as Homogeneous or Heterogeneous • Table sugar. • A mixture of table sugar and black pepper. • A mixture of sugar dissolved in water. • Oil and vinegar salad dressing. Tro's "Introductory Chemistry", Chapter 3
Practice—Classify the Following as Pure Substances or Mixtures, Continued • A homogeneous liquid whose temperature stays constant while boiling = pure substance. • Granite—a rock with several visible minerals in it = mixture. • A red solid that turns blue when heated and releases water that is always 30% of the solid’s mass = pure substance. • A gas that when cooled and compressed, a liquid condenses out but some gas remains = mixture. Tro's "Introductory Chemistry", Chapter 3
Physical Properties • Properties of an object or substance that can be measured or perceived without changing the identity of the substance
Some Physical Properties of Iron • Iron is a silvery solid at room temperature with a metallic taste and smooth texture. • Iron melts at 1538 °C and boils at 4428 °C. • Iron’s density is 7.87 g/cm3. • Iron can be magnetized. • Iron conducts electricity, but not as well as most other common metals. • Iron’s ductility and thermal conductivity are about average for a metal. • It requires 0.45 J of heat energy to raise the temperature of one gram of iron by 1°C. Tro's "Introductory Chemistry", Chapter 3
Physical Change • A change in a physical property of a substance • Same substance before and after the change
Phase Changes ArePhysical Changes • Boiling = liquid to gas. • Melting = solid to liquid. • Subliming = solid to gas. • Freezing = liquid to solid. • Condensing = gas to liquid. • Deposition = gas to solid. • State changes require heating or cooling the substance. • Evaporation is not a simple phase change, it is a solution process. Tro's "Introductory Chemistry", Chapter 3
Chemical Change • A process in which reactants are changed into one or more different products • Have breaking and making of chemical bonds
Some Chemical Properties Tro's "Introductory Chemistry", Chapter 3
Changes in Matter • Chemical Changes involve a change in the properties of matter that change its composition. • A chemical reaction. • Rusting is iron combining with oxygen to make iron(III) oxide. • Burning results in butane from a lighter to be changed into carbon dioxide and water. • Silver combines with sulfur in the air to make tarnish. Tro's "Introductory Chemistry", Chapter 3
Some Chemical Properties of Iron • Iron is easily oxidized in moist air to form rust. • When iron is added to hydrochloric acid, it produces a solution of ferric chloride and hydrogen gas. • Iron is more reactive than silver, but less reactive than magnesium. Tro's "Introductory Chemistry", Chapter 3
Changes in Matter • Changes that alter the state or appearance of the matter without altering the composition are called physical changes. • Changes that alter the composition of the matter are called chemical changes. • During the chemical change, the atoms that are present rearrange into new molecules, but all of the original atoms are still present. Tro's "Introductory Chemistry", Chapter 3
Practice—Decide Whether Each of the Observations About Table Salt Is a Physical or Chemical Property • Salt is a white, granular solid. • Salt melts at 801 °C. • Salt is stable at room temperature, it does not decompose. • 36 g of salt will dissolve in 100 g of water. • Salt solutions and molten salt conduct electricity. • When a clear, colorless solution of silver nitrate is added to a salt solution, a white solid forms. • When electricity is passed through molten salt, a gray metal forms at one terminal and a yellow-green gas at the other. Tro's "Introductory Chemistry", Chapter 3
Practice − Decide Whether Each of the Observations About Table Salt Is a Physical or Chemical Property • Salt is a white, granular solid = physical. • Salt melts at 801 °C = physical. • Salt is stable at room temperature, it does not decompose = chemical. • 36 g of salt will dissolve in 100 g of water = physical. • Salt solutions and molten salt conduct electricity = physical. • When a clear, colorless solution of silver nitrate is added to a salt solution, a white solid forms = chemical. • When electricity is passed through molten salt, a gray metal forms at one terminal and a yellow-green gas at the other = chemical. Tro's "Introductory Chemistry", Chapter 3
Practice—Classify Each Change as Physical or Chemical • Evaporation of rubbing alcohol. • Sugar turning black when heated. • An egg splitting open and spilling out. • Sugar fermenting. • Bubbles escaping from soda. • Bubbles that form when hydrogen peroxide is mixed with blood. Tro's "Introductory Chemistry", Chapter 3
Problems Decide whether the following are chemical or physical changes • Sawing a log in half • Melting chocolate in a pot on your stove • Burning your chocolate • Dissolving a nickel in acid • Cutting your hair
Different Physical Property Technique Boiling point Distillation State of matter (solid/liquid/gas) Filtration Adherence to a surface Chromatography Volatility Evaporation Density Centrifugation and decanting Separation of Mixtures • Separate mixtures based on different physical properties of the components. • Physical change. Tro's "Introductory Chemistry", Chapter 3
Distillation Tro's "Introductory Chemistry", Chapter 3
Filtration Tro's "Introductory Chemistry", Chapter 3