Naming acids and bases
140 likes | 327 Vues
Naming acids and bases. Naming Binary Acids. Binary acids contain 2 elements Steps to name: Start with “hydro” Name the element Change the ending to “ ic ” Add the word acid at the end. Binary Acid Example. Name HCl. ic. hydro. chlorine. acid. hydrochloric acid .

Naming acids and bases
E N D
Presentation Transcript
Naming Binary Acids • Binary acids contain 2 elements • Steps to name: • Start with “hydro” • Name the element • Change the ending to “ic” • Add the word acid at the end
Binary Acid Example • Name HCl ic hydro chlorine acid hydrochloric acid
Name the following… • HF • HBr • H2S • Hydrofluoric acid • Hydrobromic acid • Hydrosulfuric acid Note: sulfur is an exception, because it sounds funny otherwise
Oxyacids • Polyatomic ions that are acids • You will know it is an acid if it starts with an H
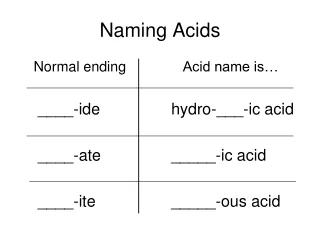
Naming acids ending in -ate • If a polyatomic ends in –ate, drop the ending and add –ic to the end. • Write the word acid after. • Example: HC2H3O2 Acid Acetate ic Acetic Acid
Name these acids • H3PO4 • HNO3 • Phosphoric Acid • Nitric Acid Phosphoric Acid is another exception, because it sounds funny
Naming acids ending in -ite • If a polyatomic ends in –ite, drop the ending and add –ous to the end. • Write the word acid after. • Example: HNO2 Acid ous Nitrite Nitrous Acid
Name these acids • H2SO3 • HClO2 • Sulfrous Acid • Chlorous Acid Remember: sulfur is an exception, because it sounds funny otherwise
Naming Bases • Bases have –OH in them • Except Ammonia (NH3) • To name: • Name the metal • Add hydroxide at the end • Example: NaOH Sodium Hydroxide