Organizing the Elements Chapter 3
150 likes | 295 Vues
Chapter 3 explores the fundamental structure of atoms, detailing their particles: protons, neutrons, and electrons. It explains how elements are identified by their unique atomic number, which corresponds to the number of protons in the nucleus. The chapter also highlights Dmitri Mendeleev's contributions to the development of the Periodic Table by organizing elements based on atomic number. Additionally, it covers the properties of metals, nonmetals, and metalloids, as well as the significance of alloys in materials science.

Organizing the Elements Chapter 3
E N D
Presentation Transcript
Intro to ATOMS • Particles of Atoms include: • Nucleus-center core • Protons-positive • Neutrons-no charge • Electrons-negative and moves rapidly (e-)
Atoms and Elements Elements identified by Number ofprotonsin the nucleus of its atom Each element has a unique atomic number-the number of protons in its nucleus Carbon – 6 Oxygen – 8 Iron – 26 However, the number of neutrons will vary Same protons, different neutrons = isotopes isotopes identified by mass number
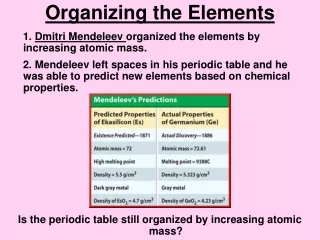
Ch. 3.2 A. Who? Dmitri Mendeleev 1. Russian scientist 2. First observed elements 3. Similar physical and chemical properties 4. Noticed patterns: Ex: Florine & Chlorine both gases/irritate lungs Ex: Silver & Copper both shiny/tarnish in air 5. Invented 1stPeriodic Table
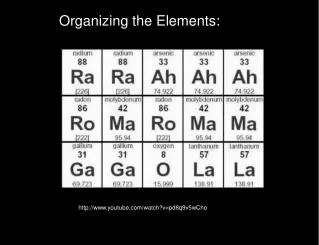
B. Today’s Periodic Table 1. Arranged by increasing atomic number 2. Atomic number = protons in the nucleus nucleus
C. Information on the Periodic Table Atomic Number 26 -elements arranged by this number Fe Atomic Symbol Iron Name of Element 55.847 Atomic Mass
Elements Song There's antimony, arsenic, aluminum, selenium, And hydrogen and oxygen and nitrogen and rhenium, And nickel, neodymium, neptunium, germanium, And iron, americium, ruthenium, uranium, Europium, zirconium, lutetium, vanadium, And lanthanum and osmium and astatine and radium, And gold and protactinium and indium and gallium, And iodine and thorium and thulium and thallium. There's yttrium, ytterbium, actinium, rubidium, And boron, gadolinium, niobium, iridium, And strontium and silicon and silver and samarium, And bismuth, bromine, lithium, beryllium, and barium. There's holmium and helium and hafnium and erbium, And phosphorus and francium and fluorine and terbium, And manganese and mercury, molybdenum, magnesium, Dysprosium and scandium and cerium and cesium. And lead, praseodymium, and platinum, plutonium, Palladium, promethium, potassium, polonium, And tantalum, technetium, titanium, tellurium, And cadmium and calcium and chromium and curium. There's sulfur, californium, and fermium, berkelium, And also mendelevium, einsteinium, nobelium, And argon, krypton, neon, radon, xenon, zinc, and rhodium, And chlorine, carbon, cobalt, copper, tungsten, tin, and sodium. These are the only ones of which the news has come to Harvard, And there may be many others, but they haven't been discovered.
C solid Hg liquid gas metals metalloid nonmetal Turn to Page 84-85 in your book and color/copy the table like the one in your book
Families (Groups) and Periods Vertical: Families/Groups (columns) are have similar properties Horizontal: Periods have series of different elements not alike
Properties of Metals 1. Hardness 2. Shininess 3. Malleability -pounded into shape 4. Ductility -pulledout into a longwire 5. Reactivity -combines or reacts to other metals
Alloys • A mixture of metals • Brass = copper + zinc • Bronze = copper + tin • Stainless Steel = carbon + chromium + vanadium
Properties of Nonmetals Nonmetals-lack most of the properties of metals 1. Dull 2. Brittle 3. Low densities 4. Poor Conductors of heat and electricity
Properties of Metalloids 1. Some characteristics of both metals and nonmetals 2. Most common-silicon 3. Semiconductor-ability to carry electricity • Used for computer chips, transistors, lasers