Naming Acids and Bases
270 likes | 980 Vues
Naming Acids and Bases. Naming Inorganic Acids. inorganic acids-have one (monoprotic) or more (di-, tri-, poly-protic) ionizable hydrogen atoms in chemical formula. dissociates in solution to produce H+ ions. examples: HCl, H 2 S, HClO 3. Naming Inorganic Acids.

Naming Acids and Bases
E N D
Presentation Transcript
Naming Inorganic Acids • inorganic acids-have one (monoprotic) or more (di-, tri-, poly-protic) ionizable hydrogen atoms in chemical formula dissociates in solution to produce H+ ions examples: HCl, H2S, HClO3
Naming Inorganic Acids There are two types of inorganic acids: binary and oxyacids
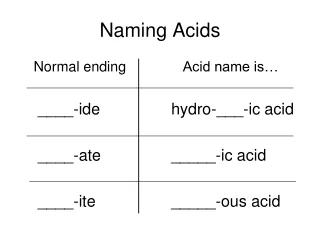
Naming Inorganic Acids • Must remember the names of polyatomic and monatomic anions and the whole concept of formula writing • extension of ionic (stock) nomenclature • acid names are based on suffix of anion
Naming Inorganic Acids *The number of hydrogen atoms is exactly equal to the numerical value of the charge on the anion
Naming Organic Acids • remember IUPAC organic nomenclature (meth-, eth-, prop-, but-...) (-ane, -ene, -yne) • carboxylic acid group is the functional group that makes organic compounds acidic (-COOH) • the carbon of this group counts for one of the carbons in the name, then ignore this group when determining -ane, -ene, -yne. • general formula: CxHyCOOH or CH3CH2CH2...COOH
Naming Organic Acids example: C2H5COOH or CH3CH2COOH total of carbon atoms = 3, prop hydrogen atoms are one less than 2n+2 = ane COOH shows acid group = drop final “e,” add -oic acid NAME: propanoic acid Remember that hydrogen with the -COOH group does NOT count for number of hydrogen
Naming Organic Acids example: C3H7COOH or CH3CH2CH2COOH total of carbon atoms = 4, but hydrogen atoms are one less than 2n+2 = ane COOH shows acid group = drop final “e,” add -oic acid NAME: butanoic acid
Try these... Name the following organic acids CH3COOH C5H11COOH solutions: 1. ethanoic acid; 2. hexanoic acid
Naming Organic Acids example: C2H3COOH or CH2=CHCOOH total of carbon atoms = 3, prop hydrogen atoms are one less than 2n = ene COOH shows acid group = drop final “e,” add -oic acid NAME: propenoic acid
Try these.... Name the following organic acids C5H9COOH C3H3COOH HCOOH solutions: 1. hexenoic acid; 2. butynoic acid; 3. methanoic acid
Naming Inorganic Bases • inorganic bases - compounds that have one (monobasic) or more (di-, tri-, or poly-basic) hydroxide ions in their formula • hydroxide ions will dissociate in solution to produces OH-
Naming Inorganic Bases • all inorganic bases are hydroxides-you should already know how to name these general form: M(OH)n examples: NaOH, Ca(OH)2, Al(OH)3
Naming Organic Bases • organic bases - compounds that have one (monobasic) or more (di-, tri-, or poly-basic) hydroxide ions in their formula largely comprised of hydrocarbons • the functional group that makes organic compounds basic is the amine group (nitrogen w/ hydrogen)
Naming Organic Bases • have nitrogen atoms written last with one or two hydrogen atoms • recall IUPAC nomenclature • nitrogen needs three bonds • general form: CxHyN or CxHyNH or CxHyNH2
Naming Organic Bases example: C2H5NH2 total of carbon atoms = 2, eth hydrogen atoms are one less than 2n+2 = ane NH2 shows base group = drop final “e,” add amine NAME: ethanamine
Naming Organic Bases example: C4H9NH2 total of carbon atoms = 4, but hydrogen atoms are one less than 2n+2 = ane NH2 shows base group = drop final “e,” add amine NAME: butanamine
Naming Organic Bases example: C3H5NH2 total of carbon atoms = 3, prop hydrogen atoms are one less than 2n = ene NH2 shows base group = drop final “e,” add amine NAME: propenamine
Naming Organic Bases example: C5H7NH2 total of carbon atoms = 5, pent hydrogen atoms are one less than 2n-2 = yne NH2 shows base group = drop final “e,” add amine NAME: pentynamine
Try these... C2HNH2 C6H9NH2 C10H21NH2 C9H17NH2 solutions: 1. ethynamine; 2. hexynamine; 3. decanamine; 4. nonenamine
Naming Organic Bases example: (CH3)2NH total of carbon atoms = 1 taken twice, dimethyl NH shows base group = add amine NAME: dimethylamine
Naming Organic Bases example: (C2H5)3N total of carbon atoms = 2 taken thrice, triethyl NH shows base group = add amine NAME: triethylamine
Try these... (CH3)3N (C2H5)2NH solutions: 1. trimethylamine; 2. diethylamine
Naming Organic Bases • 2 small exceptions: H2NNH2 hydrazine:no carbon atoms, 2 amine groups NH3 ammonia: no carbon atoms, 1 amine with an extra hydrogen